The Common European Submission Platform (CESP) system was designed to allow for a single, electronic submission for clinical trials to all European regulatory agencies, as required. The single-platform submission aims reduce the administrative burden of set-up in multi-national trials through automated-distribution of the application with no CD/DVD or ‘hard copy’ requirement.
Initial registration
To submit via CESP you need access to the system. You should consider who within your department will require access in order to submit new applications, amendments, DSURs etc, as well as ensuring contingencies are in place should staff members be unavailable for any reason.
To register with CESP, please contact ctrg@admin.ox.ac.uk in the first instance to request a CESP account. We will need a registration name (first name, last name) and preferred email address. This can be a generic email address monitored by multiple users and the name of the individual user or clinical trial. Currently, all University of Oxford users are registered under a single ‘Company’. We may in future, dependent on the functionality and development of the CESP system, register each department as individual ‘sub-companies’ under the University umbrella.
Following your registration you will receive an email to the registered address. This will include:
- a link to the CESP web platform
- contact details and opening hours for the CESP support team
- your CESP username (formatted as: c3716-surname/first initial – for example, c3716-bloggsj)
In order to complete your registration, follow the link in the email to the CESP log-in page where you will be required to set your password. If you do not receive this email please check your junk email folder. The password link will time out and demands prompt action.
CESP 2.0 requires Internet Explorer 10+, Google Chrome, Mozilla Firefox or Safari.
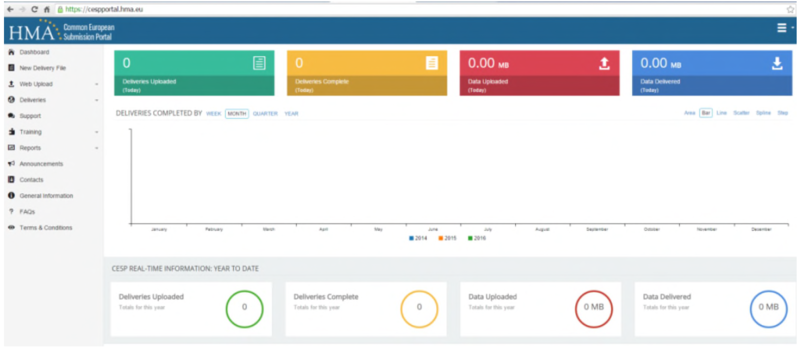
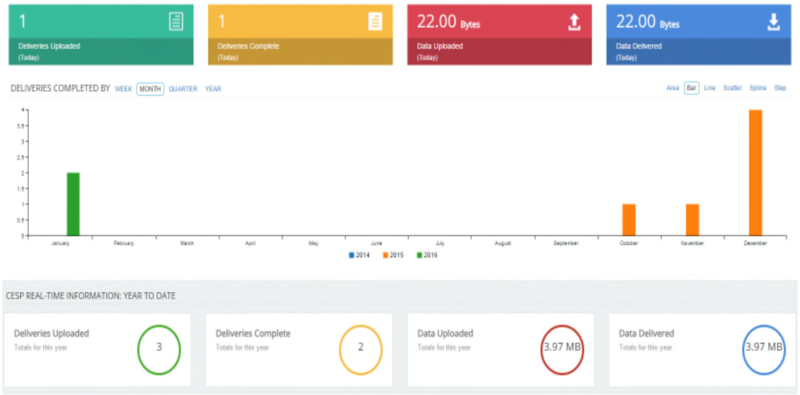
Upon log-in you will be presented with your ‘Dashboard’ (shown below). This will appear empty, but following CESP uploads it will allow you to track the number and size of submissions from your account through the system.
Using CESP to submit
CESP allows for electronic submission of clinical trial applications to the appropriate regulatory agency, Europe-wide.
The submission should be a single .zip file. This package should include all the documentation required for the submission. CESP offer guidance on the creation of .zip folders using standard Windows compression.
When naming your .zip file, and the files within the .zip package, please use the standard ASCII character set. Do not use any special characters. File names and paths should not exceed 240 characters.
All submissions also require a covering letter. The letter must include the CESP submission number: this is the number of the downloaded .xml file.
Delivery files
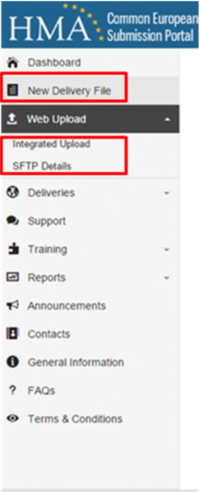
The first step in the submission process is to create a new ‘Delivery File’. Please select this option from your sidebar and proceed through the four steps.
Step 1 will require you to indicate the ‘Area’, ‘Activity’ and ‘Sub-Activity’ of the submission from the drop-down list provided. This should largely be intuitive based on the type of submission you are intending, but please contact either the CESP support team or CTRG for further guidance if you are unsure of your activity.
Step 2 will ask for your ‘Procedure Type’. This is in reference to the method by which you will be gaining marketing authorisation, should that be your intention.
You will also be asked to select your ‘Submission Type’. Unless you are aware this is not the case, please select ‘Other eSubmission Type’ (which would not have been ‘Technically Validated’).
Finally you can select the relevant competent authorities to receive your submission and any additional email address to be included in the submission verification.
Following the completion of these steps, a .xml file will be available for download. Save this to a safe location as it will need to be uploaded alongside your .zip file.
Please note that delivery files cannot be renamed and each is unique to a single submission. The number in the file name is your CESP submission number and should be included in your covering letter.
The FAQs section of the website has a specific section for delivery files if you require support. As CESP is designed for a variety of applications outside of clinical trials, there will be numerous acronyms and options which will not be relevant to your research. If you require further support, please contact CTRG.
Upload
There are two methods by which a submission can be made:
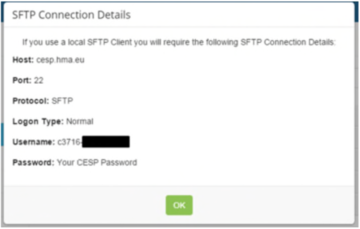
- via the ‘SFTP’ or Secure File Transfer Protocol
- via the online ‘Integrated Upload’ option